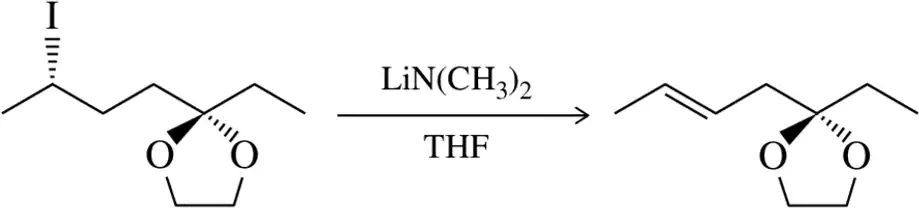
Predict the major product(s) of the following elimination reactions, paying close attention to the stereochemical outcome of the reactions.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 9:36m
9:36mMaster Drawing the E2 Mechanism. with a bite sized video explanation from Johnny
Start learning