Design a synthesis for each of the following, using an intramolecular reaction:
d.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
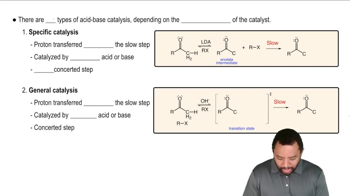
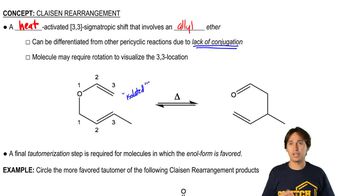

 6:30m
6:30mMaster Friedel-Crafts Alkylation with a bite sized video explanation from Johnny
Start learning