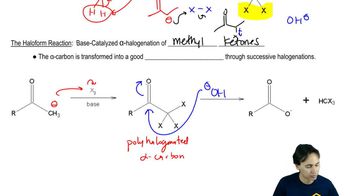
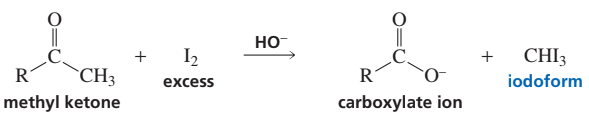
Predict the products of the following reactions.
(a) cyclopentyl methyl ketone + excess Cl2 + excess NaOH
(b) 1-cyclopentylethanol + excess I2 + excess NaOH
(c) propiophenone + excess Br2 + excess NaOH
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: