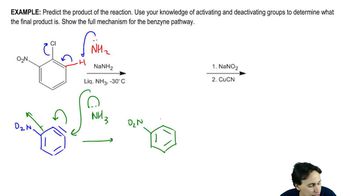
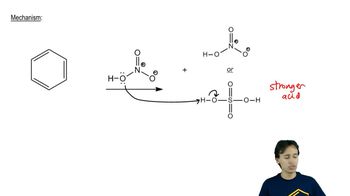
A mixture of 0.10 mol benzene and 0.10 mol p-xylene was allowed to react with 0.10 mol nitronium ion until all the nitronium ion was gone. Two products were obtained: 0.002 mol of one and 0.098 mol of the other.
b. Why was more of one product obtained than of the other?