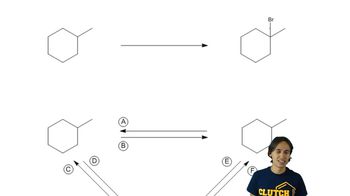
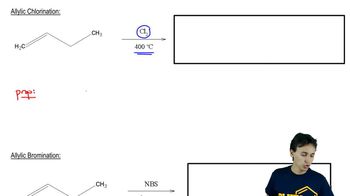
When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane.
b. How would you run this reaction to get a good conversion of methane to CH3Cl? Of methane to CCl4?