Show how you would synthesize octanal from each compound. You may use any necessary reagents.
(d) 1-bromoheptane
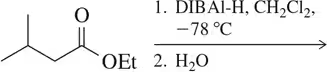
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:07m
2:07mMaster Why LiAlH4 doesn't work with a bite sized video explanation from Johnny
Start learning