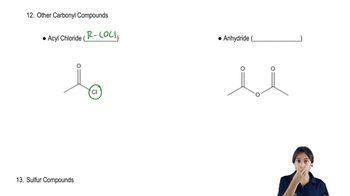
Grignard reagents add to carbonate esters as they add to other esters.
(c) Diethyl carbonate is a liquid reagent that is easy to handle. Also show how you might use diethyl carbonate instead of methyl isocyanate to make Sevin® insecticide.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: