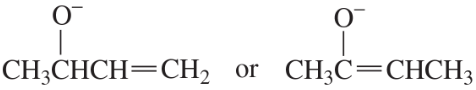The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
a. Use resonance forms to show the delocalization (over four carbon atoms) of the positive charge of the benzyl cation.