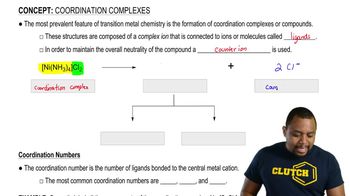
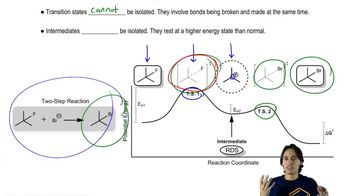
Draw a reaction coordinate diagram, making sure to label reactants (R), products (P), intermediates (I), transition states (‡), activation energies ( Ea) , and ∆G°, for each of the following.
(b) an exothermic, two-step reaction where the second step is rate-determining