For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy.
(c) [H2COCH3]+
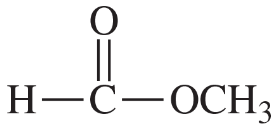
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:34m
3:34mMaster The rules you need for resonance: with a bite sized video explanation from Johnny
Start learning