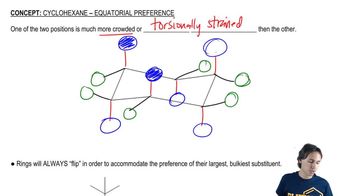
For each chair on the left, place the substituents on the flipped chair. [Recall that the axial/equatorial designation changes from one chair to the next, but the carbon to which the substituent is attached does not.]
(f)
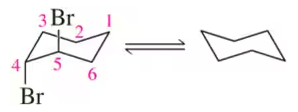
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: