Textbook Question
Which of the following objects are chiral?
e. a wheelbarrow
f. a remote control device
g. a nail
h. screw
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:25m
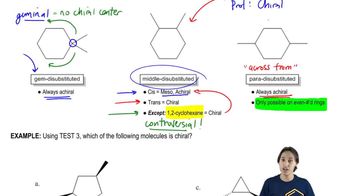
1:25mMaster How and when to use the internal line of symmetry test. with a bite sized video explanation from Johnny
Start learning