a. Which of the following reactions does not give the carbonyl product shown?
b. Which of the reactions that do not occur can be made to occur if an acid catalyst is added to the reaction mixture?
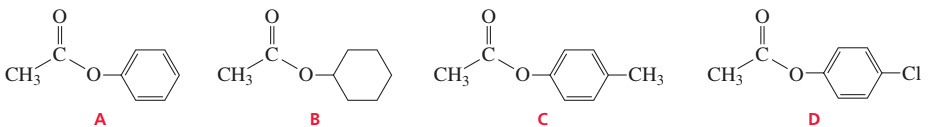
9.
10.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 9:32m
9:32mMaster NAS - The Three Rules with a bite sized video explanation from Johnny
Start learning