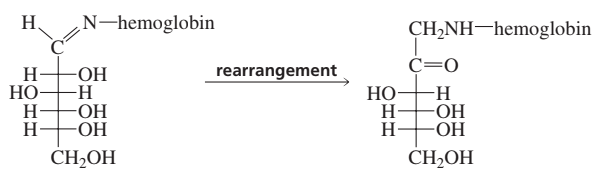
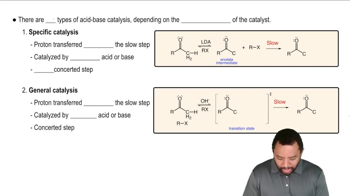
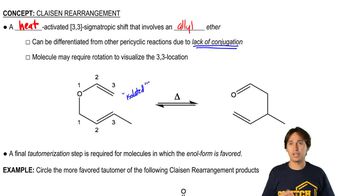
For practice in recognizing mechanisms, classify each reaction according to the type of mechanism you expect:
1. Free-radical chain reaction
2. Reaction involving strong bases and strong nucleophiles
3. Reaction involving strong acids and strong electrophiles.
(d)