Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (i) Br2 (ii) Cl2
(f)
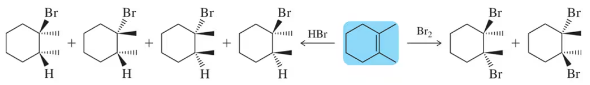
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:27m
2:27mMaster General properties of halogenation. with a bite sized video explanation from Johnny
Start learning