List three different sets of reagents (each set consisting of a carbonyl compound and a Grignard reagent) that could be used to prepare each of the following tertiary alcohols:
b.
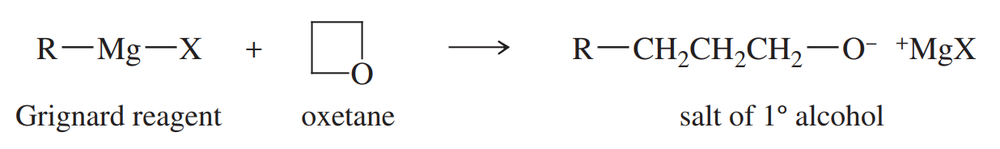
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: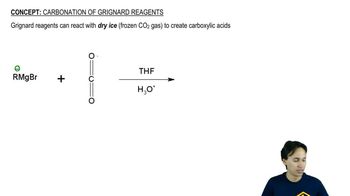


 13:4m
13:4mMaster Reactions of Organometallics with a bite sized video explanation from Johnny
Start learning