Textbook Question
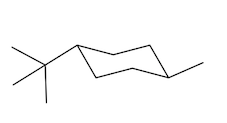
For each of the following compounds, determine whether the cis isomer or the trans isomer is more stable.
a.
b.
c.



 Verified step by step guidance
Verified step by step guidance
 4:02m
4:02mMaster Equatorial Preference with a bite sized video explanation from Johnny
Start learning
