Textbook Question
Would the following nucleophiles be more likely to participate in an SN1 or SN2 reaction?
(d) NH3
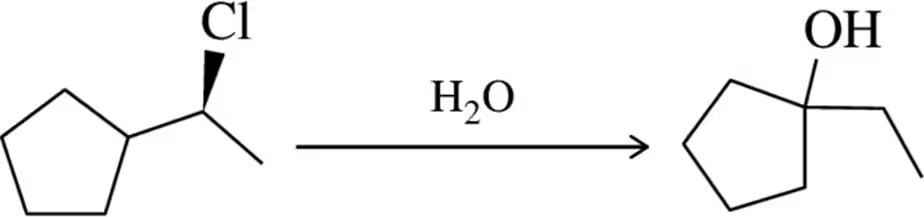
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:32m
3:32mMaster How do we predict if the mechanism is SN1 or SN2? with a bite sized video explanation from Johnny
Start learningFor each solvent, indicate the most likely substitution reaction to take place.
(b)
For each solvent, indicate the most likely substitution reaction to take place.
(d)