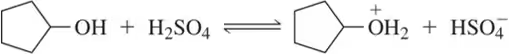
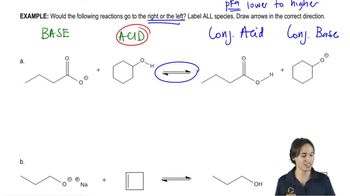
Write equations for the following acid–base reactions. Label the conjugate acids and bases, and show any inductive stabilization. Predict whether the equilibrium favors the reactants or products. Try to do this without using a table of pKa values, but if you need a hint, you can consult Appendix 4.
g. NaOCH2CH3 + Cl2CHCH2OH
h. H2Se + NaNH2
i. CH3CHFCOOH + FCH2CH2COO–