Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
f. 1-(phenylmethyl)cyclohexanol
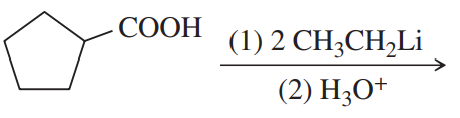
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 0:24m
0:24mMaster Intro to Predict the Product with a bite sized video explanation from Johnny
Start learning