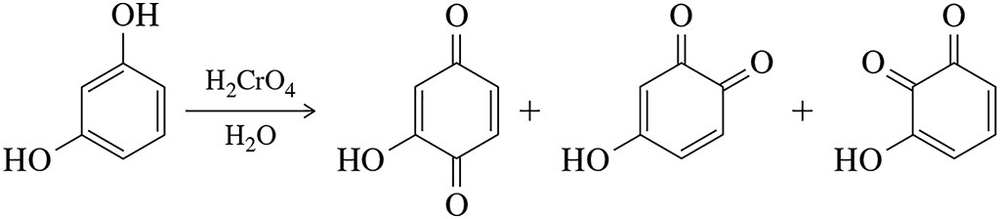
We explain in Chapter 24 that bisphenols can be oxidized to quinones.
(a) Calculate the oxidation numbers of C1 and C₂ in going from reactant to product.
(b) Provide a mechanism for this transformation. [The reaction begins like the alcohol oxidations of Section 13.9.]