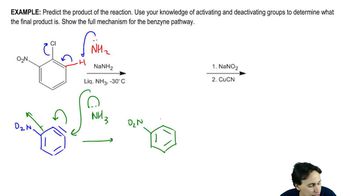
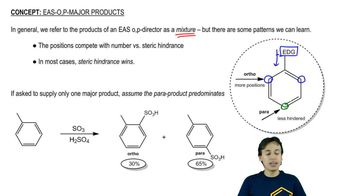
Show how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures.
b. 1-phenyl-1-methoxybutane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:49m
2:49mMaster Aromatic synthesis starting with benzene/benzene derivatives with a bite sized video explanation from Johnny
Start learning