Show how you would synthesize the following compound from alkyl halides, vinyl halides, and aryl halides containing no more than six carbon atoms.
(a) octane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: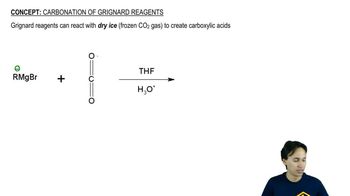


 13:4m
13:4mMaster Reactions of Organometallics with a bite sized video explanation from Johnny
Start learning