Textbook Question
Which has the greater delocalization energy?
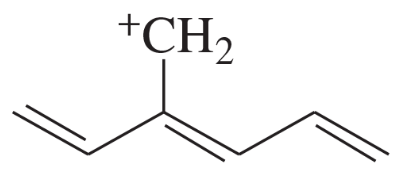
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:31m
4:31mMaster Stability of Conjugated Intermediates with a bite sized video explanation from Johnny
Start learning