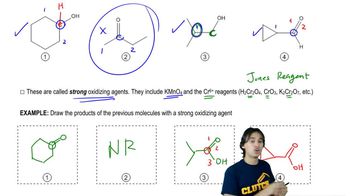
Which of the following compounds would give a positive Tollens test? (Remember that the Tollens test involves mild basic aqueous conditions.)
(a) CH3CH2CH2COCH3
(b) CH3CH2CH2CH2CHO
(c) CH3CH=CHCH=CHOH
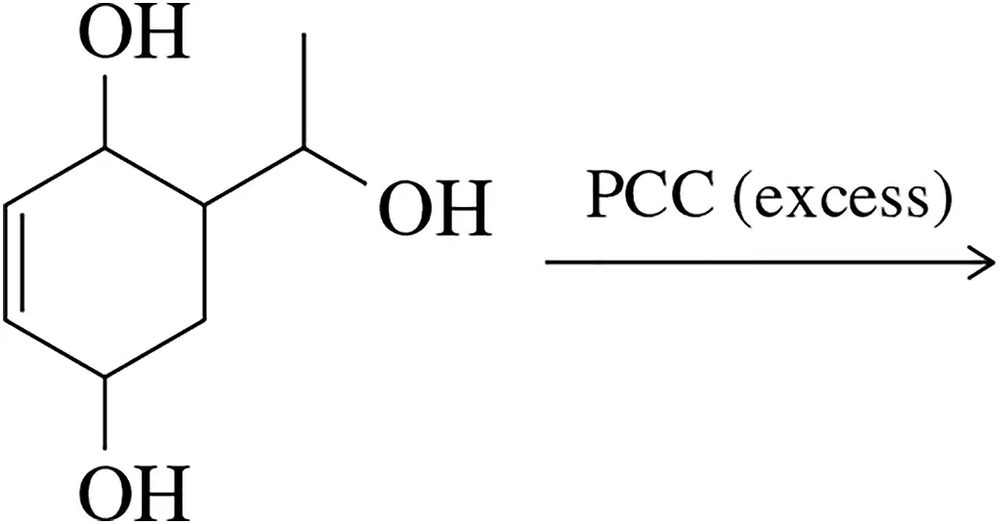
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: