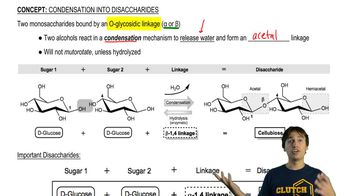
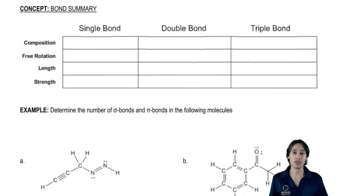
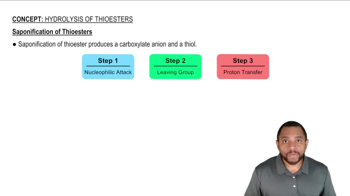
In each of the disaccharides shown (a–c), (i) identify the reducing end of the sugar, (ii) tell what monosaccharides are used to make it, and (iii) tell what type of glycoside linkage is present.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: