Textbook Question
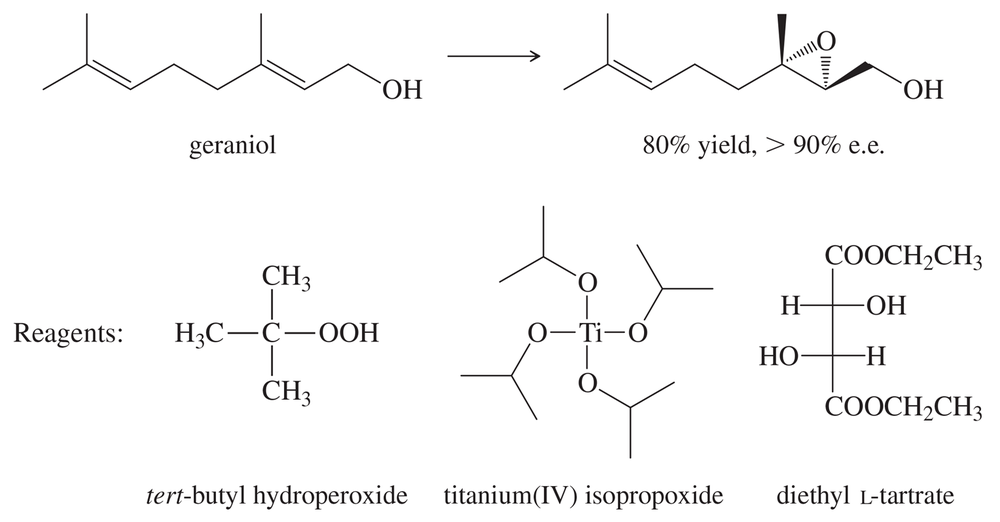
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:39m
4:39mMaster Important Reagents of Sharpless Epoxidation. with a bite sized video explanation from Johnny
Start learning