Imagine a sample that is enriched in the R enantiomer. If the % ee of the sample is 83%,
(a) what percent of the mixture is racemic?
(b) What is the ratio of R to S?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: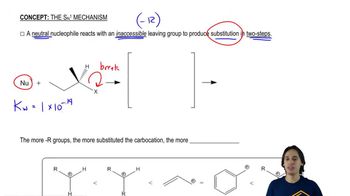
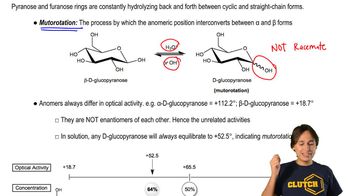
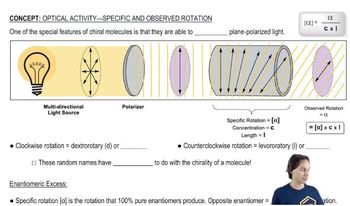

 3:23m
3:23mMaster How to solve for the percentage of each enantiomer. with a bite sized video explanation from Johnny
Start learning