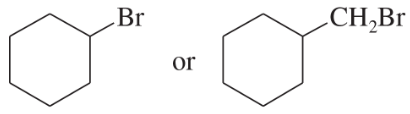
A reluctant first-order substrate can be forced to ionize by adding some silver nitrate (one of the few soluble silver salts) to the reaction. Silver ion reacts with the halogen to form a silver halide (a highly exothermic reaction), generating the cation of the alkyl group.
Give mechanisms for the following silver-promoted rearrangements.
(a)