Multiple Choice
Identify the type of following pericyclic reaction.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: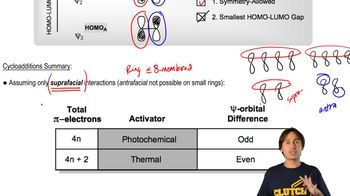
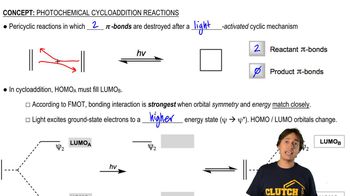

 7:37m
7:37mMaster Properties and Types of Pericyclic Reactions with a bite sized video explanation from Johnny
Start learning