Provide the expected product for the reaction of each of the following alkenes with H2SO4 and H2O.
(d)
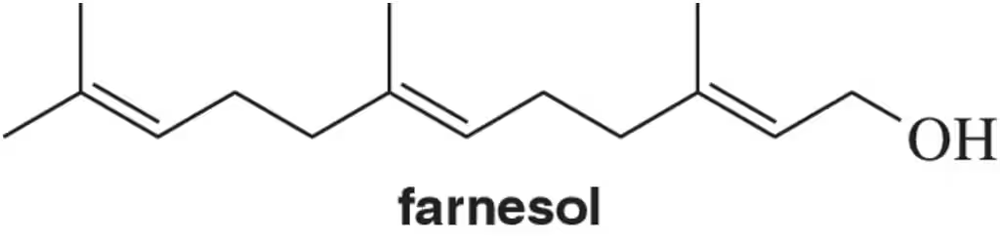
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:32m
6:32mMaster General properties of acid-catalyzed hydration. with a bite sized video explanation from Johnny
Start learning