Identify the resonance structure that will be produced given the molecule shown and the electron flow indicated.
(a)
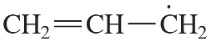
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:34m
3:34mMaster The rules you need for resonance: with a bite sized video explanation from Johnny
Start learning