How can the following compounds be prepared using ethyne as the starting material?
f.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
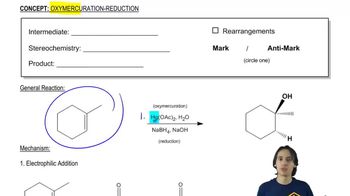
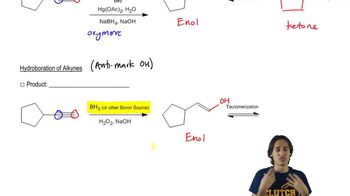

 0:48m
0:48mMaster The definition of hydrogenation. with a bite sized video explanation from Johnny
Start learning