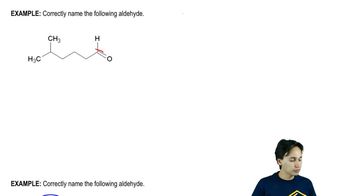
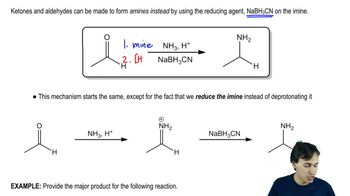
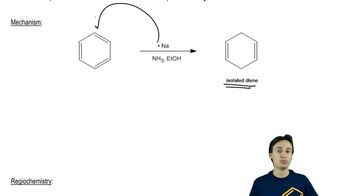
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(c) Ph-COOH
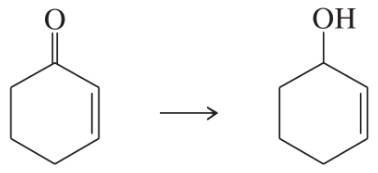
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: