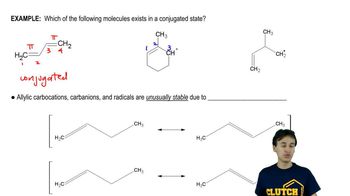
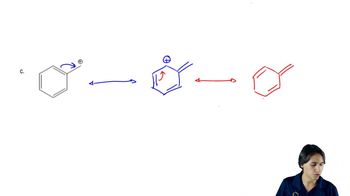
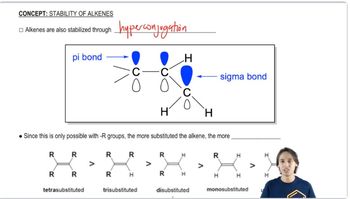
Using Table 7-2 as a guide, predict which member of each pair is more stable, as well as by about how many kJ/mol or kcal/mol.
a. cis,cis-hexa-2,4-diene or trans,trans-hexa-2,4-diene
b. 2-methylbut-1-ene or 3-methylbut-1-ene
c. 2-methylbut-1-ene or 2-methylbut-2-ene
d. cis-4-methylpent-2-ene or 2-methylpent-2-ene