Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the following compounds.
(c) 4-methylpentan-2-ol
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: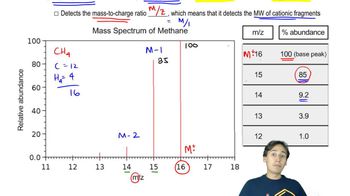

 4:28m
4:28mMaster Ionization Potentials with a bite sized video explanation from Johnny
Start learning