(a) Give the products expected when (+)-glyceraldehyde reacts with HCN.
(b) What is the relationship between the products? How might they be separated?
(c) Are the products optically active? Explain.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: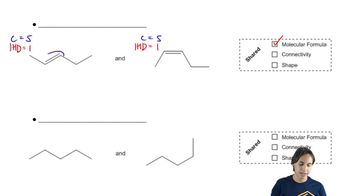
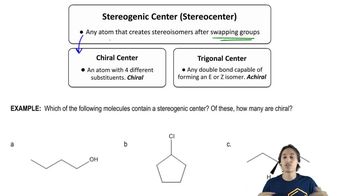

 3:51m
3:51mMaster Determining when molecules are different. with a bite sized video explanation from Johnny
Start learning