Open Question
Beginning from phenol, determine the chemical steps needed to prepare the following compound.
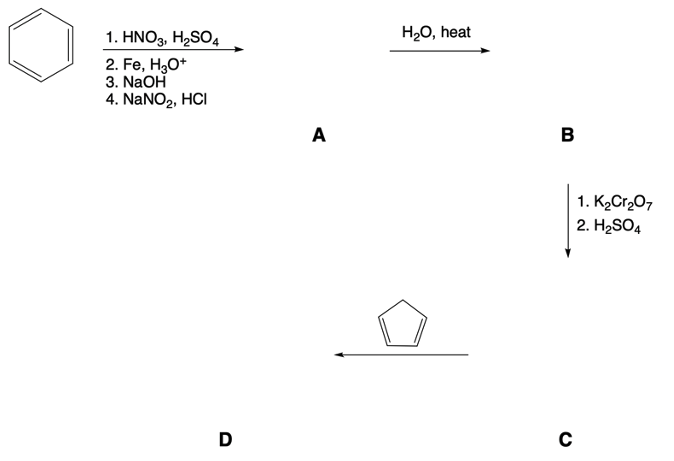
 Verified step by step guidance
Verified step by step guidance
 1:47m
1:47mMaster Oxidation of Phenols to Quinones Concept 1 with a bite sized video explanation from Johnny
Start learning
