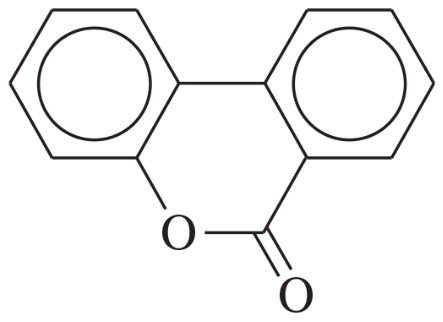
Predict the major products of bromination of the following compounds, using Br2 and FeBr3 in the dark.
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:29m
4:29mMaster Activity and Directing Effects with a bite sized video explanation from Johnny
Start learning