Use the four compounds shown below to answer the following questions:
b. Why is o-fluorobenzoic acid the weakest of the ortho-halo-substituted benzoic acids?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: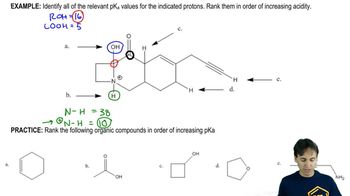


 5:39m
5:39mMaster Aromatic hydrocarbon acidity with a bite sized video explanation from Johnny
Start learning