Open Question
Determine compounds A and B from the following reaction sequence.
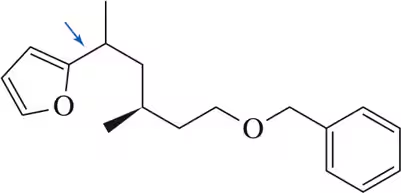
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:14m
3:14mMaster Negishi Coupling Reaction with a bite sized video explanation from Johnny
Start learning