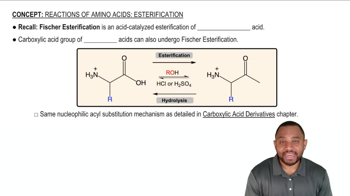
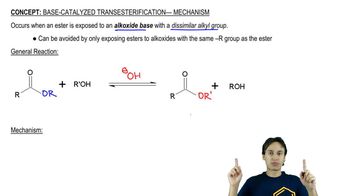
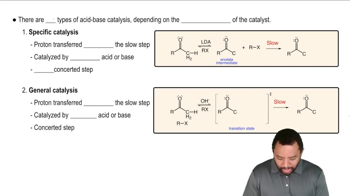
Show how you would use anhydrides to synthesize the following compounds. In each case, explain why an anhydride might be preferable to an acid chloride.
(a) n-octyl formate
(b) n-octyl acetate
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: