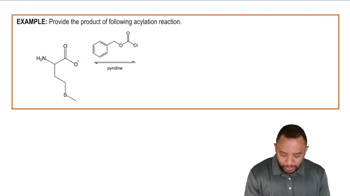
Show how to synthesize the following amines from the indicated starting materials by acylation–reduction.
(a) N-butylpiperidine from piperidine
(b) N-benzylaniline from aniline
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: