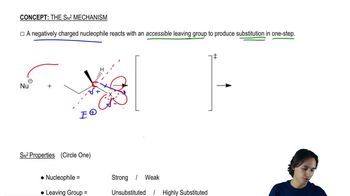
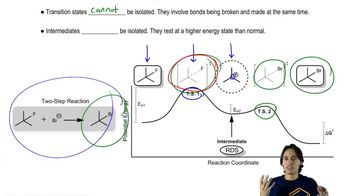
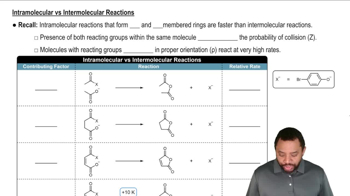
The rate of the reaction of methyl iodide with quinuclidine was measured in nitrobenzene, and then the rate of the reaction of methyl iodide with triethylamine was measured in the same solvent. The concentration of the reagents was the same in both experiments.
b. The same experiment was done using isopropyl iodide instead of methyl iodide. Which reaction had the larger rate constant?