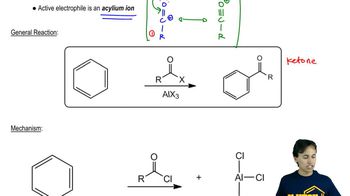
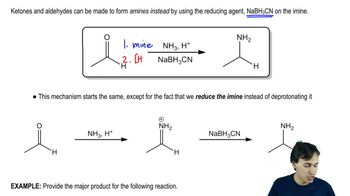
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
f. 1-phenyl-2,2-dimethylpropane
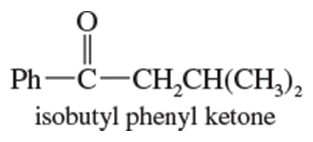
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: