For each Fischer projection, label each asymmetric carbon atom as (R) or (S).
(a)
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: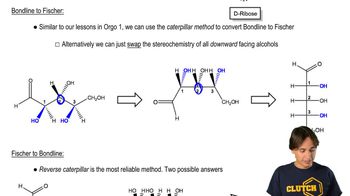
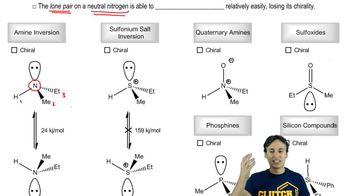
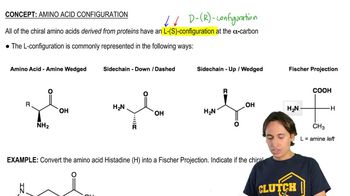

 2:32m
2:32mMaster R and S rule for Fischer Projections. with a bite sized video explanation from Johnny
Start learning