Predict the characteristic infrared absorptions of the functional groups in the following molecules.
(d) pent-1-yne
(e) diethylamine
(f) pentanoic acid
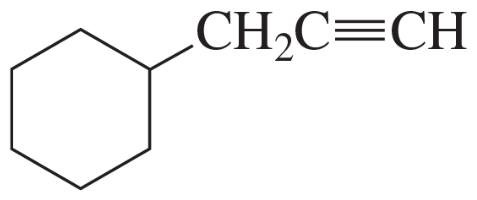
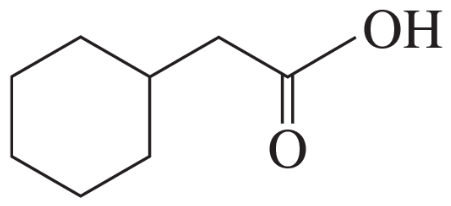
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: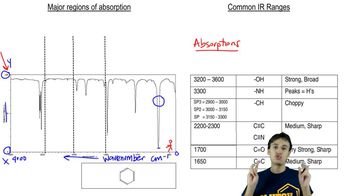


 16:47m
16:47mMaster Common IR Frequencies with a bite sized video explanation from Johnny
Start learning