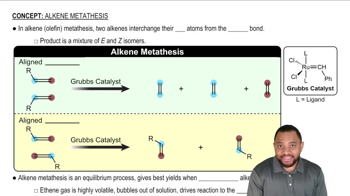
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
d. a mixture of osmic acid and hydrogen peroxide
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: