Rank each group of compounds from most reactive to least reactive toward electrophilic aromatic substitution:
e. p-methylnitrobenzene, 2-chloro-1-methyl-4-nitrobenzene, 1-methyl-2,4-dinitrobenzene, p-chloromethylbenzene
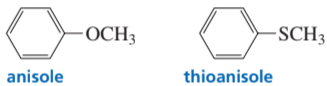
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:29m
4:29mMaster Activity and Directing Effects with a bite sized video explanation from Johnny
Start learning