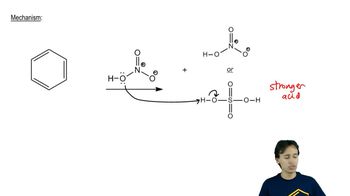
Formation of the carbocation should be fastest for which leaving group?
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 3:06m
3:06mMaster How to use the factors affecting acidity to predict leaving group ability. with a bite sized video explanation from Johnny
Start learning